Host Kevin Patton revisits the concept of using the syllabus and other course documents to build a positive and productive course culture. Poop—it's everywhere! Does the font or typeface we use affect students—especially regarding learning and memory? We look for answers in this episode!
00:00 | Introduction
00:52 | Revisiting the Syllabus
16:28 | Poop. Poop. Poop.
19:00 | Sponsored by AAA
19:59 | Fonts Are Important in Teaching & Learning
30:54 | Sponsored by HAPI
31:57 | Desirably Difficult Reading?
42:00 | Sponsored by HAPS
43:00 | Fluent & Dysfluent Fonts
56:12 | Staying Connected
★ If you cannot see or activate the audio player, go to: theAPprofessor.org/podcast-episode-123.html
🏅 Apply for your credential (badge/certificate) for listening to this episode: theAPprofessor.org/podcast-episode-123.html/#badge
❓ Please take the anonymous survey: theAPprofessor.org/survey
☝️ Questions & Feedback: 1-833-LION-DEN (1-833-546-6336)
✔️ Follow The A&P Professor on Twitter, Facebook, Blogger, Revue, Tumblr, or Instagram! @theAPprofessor
📰 Get the thrice-weekly TAPP Science & Education Updates theAPprofessor.org/updates
Typography must often draw attention to itself before it will be read. Yet in order to be read, it must relinquish the attention it has drawn. (Robert Bringhurst)
Revisiting the Syllabus
15.5 minutes
Creating and nurturing a course culture can be influenced by our syllabus and other course materials. We revisit this idea with a few more tips and tweaks.
★ Anatomy &; Physiology Syllabus: It's an Art | TAPP 120
★ Are We Answering Student Questions? | Science Updates | TAPP 92
★ Wendy Riggs has a huge collection of anatomy, physiology, and general bio, instructional videos she uses in her flipped classes youtube.com/user/wendogg1
★ Natalie Wade has engaging short videos about A&P content and study tips at The Anatomy Gal youtube.com/c/TheAnatomyGal
★ Jamie Chapman has a collection (Chapman Histology) of short (under 3 minutes) videos guiding students through lessons in histology youtube.com/c/ChapmanHistology
Poop. Poop. Poop.
2.5 minutes
After releasing The Poop Episode | Using Fecal Changes to Monitor Health | TAPP 121, I learned of a whole movement of poop listening on smart speakers. And that there are actually poop songs that are viral hits. Really.
★ When kids yell 'Alexa, play poop,' you'll hear these songs (story from All Things Considered on National Public Radioo) AandP.info/wv2
★ The Foot Book (Bright & Early children's book by Dr. Seuss; can be read as The Poop Book) geni.us/afvGc
★ CHOC Stool Diary AandP.info/4yq
★ Bowel Symptom Journal (from Alberta Health Services) AandP.info/6fw
★ Poop Apps: 5 Tools for Tracking Your Stools AandP.info/5ow
Sponsored by AAA
56 seconds
A searchable transcript for this episode, as well as the captioned audiogram of this episode, are sponsored by the American Association for Anatomy (AAA) at anatomy.org.
Don't forget—HAPS members get a deep discount on AAA membership!
AMERICAN ASSOCIATION FOR ANATOMY STATEMENT OF RESPONSIBILITY FOR ITS HISTORY OF RACISM (Press release from AAA, giving the full text of the statement) AandP.info/eei
Fonts Are Important in Teaching & Learning
11 minutes
At the suggestion of listener Dr. David Curole, we examine the roles that different fonts can play in teaching, learning, and memory. This segment reviews some past discussions of fonts, then introduces some new concepts of using fonts in teaching. Featured is a Word Dissection of the terms fluent font and dysfluent (disfluent) font.
★ Communication, Clarity, & Medical Errors | Episode 55
★ Anatomy & Physiology Syllabus: It's an Art | TAPP 120
★ Why Anatomy & Physiology Students Need Sectional Anatomy | TAPP 116
Sponsored by HAPI Online Graduate Program
59 seconds
The Master of Science in Human Anatomy & Physiology Instruction—the MS-HAPI—is a graduate program for A&P teachers, especially for those who already have a graduate/professional degree. A combination of science courses (enough to qualify you to teach at the college level) and courses in contemporary instructional practice, this program helps you be your best in both on-campus and remote teaching. Kevin Patton is a faculty member in this program at Northeast College of Health Sciences. Check it out!
Desirably Difficult Reading?
10 minutes
The article How Fonts Affect Learning and Memory by Carla Delgado takes our conversation a step further by looking the potential role of dysfluent fonts in learning.
★ How Fonts Affect Learning and Memory (article in Discover Magazine by Carla Delgado mentioned in this segment) AandP.info/wof
★ A Review of the Cognitive Effects of Disfluent Typography on Functional Reading (review article from The Design Journal) AandP.info/mwt
★ Fortune Favors the Bold (and the Italicized): Effects of Disfluency on Educational Outcomes (article from Proceedings of the Annual Meeting of the Cognitive Science Society) AandP.info/jjt
★ Changing Fonts in Education: How the Benefits Vary with Ability and Dyslexia (article from The Journal of Educational Research) AandP.info/yt4
★ Fluency and the Detection of Misleading Questions: Low Processing Fluency Attenuates the Moses Illusion (article from the journal Social Cognition) AandP.info/jul
Sponsored by HAPS
56 seconds
The Human Anatomy & Physiology Society (HAPS) is a sponsor of this podcast. You can help appreciate their support by clicking the link below and checking out the many resources and benefits found there. Watch for virtual town hall meetings and upcoming regional meetings!
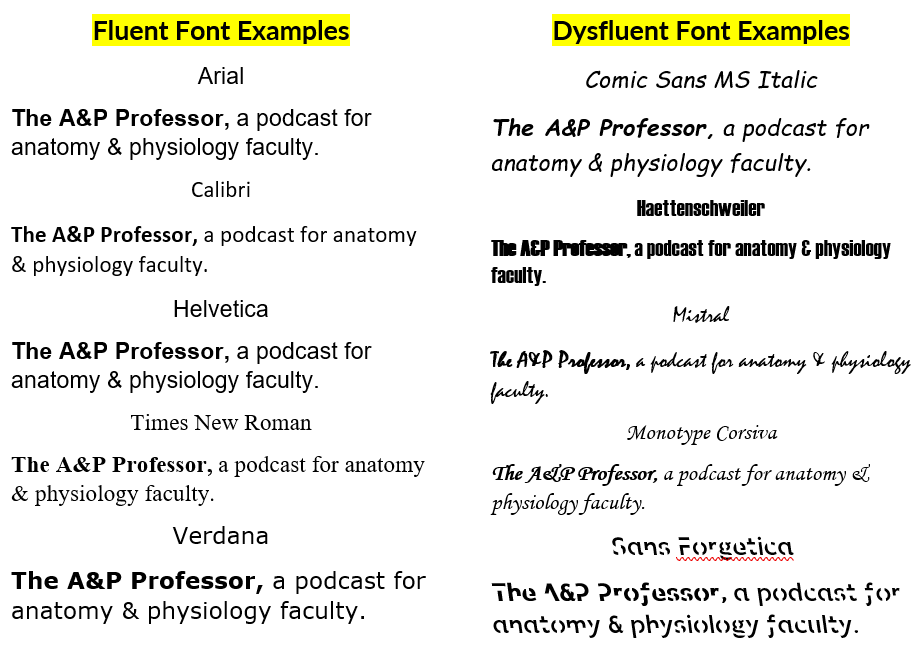
Fluent & Dysfluent Fonts
13 minutes
We identify some potentially fluent fonts, as well as a few dysfluent fonts (see image below or at AandP.info/ihy). Sans Forgetica font was developed specifically to be dysfluent in a way that promotes remembering what is read. Does it work? Should we incorporate dysfluent fonts in our teaching materials?
★ Fonts and Fluency: The Effects of Typeface Familiarity, Appropriateness, and Personality on Reader Judgments (thesis by Tim Wang) AandP.info/0hf
★ Previously claimed memory boosting font 'Sans Forgetica' does not actually boost memory (story from ScienceDaily) AandP.info/zp4
★ The science of Sans Forgetica - The font to remember (video from the creators of Sans Forgetica) AandP.info/ox5
★ An unforgettable year – Sans Forgetica turns one (article from the RMIT University website) AandP.info/fo3
★ Sans Forgetica: Study Mode by RMIT University (plugin for Chrome browser lets you read any web page in Sans Forgetica) AandP.info/fc3
★ Sans Forgetica (free download for personal use) AandP.info/o4g
★ Can very small font size enhance memory? (article from journal Memory & Cognition) AandP.info/rlk
★ Sans Forgetica is not desirable for learning (article from the journal Memory) AandP.info/hmu
★ The role of font size and font style in younger and older adults' predicted and actual recall performance (article from
Neuropsychology, development, and cognition. Section B, Aging, Neuropsychology and Cognition) AandP.info/r6s
People
Contributors: David Curole, Terry Thompson
Mentions: Wendy Riggs, Natalie Wade, Jaime Chapman, Robert Bringhurst, Carla Delgado
Production: Aileen Park (announcer), Andrés Rodriguez (theme composer, recording artist), Rev.com team (transcription), Kevin Patton (writer, editor, producer, host)
If the hyperlinks here are not active, go to TAPPradio.org to find the episode page.
★ More details at the episode page: theAPprofessor.org/podcast-episode-123.html
★ Transcript available in the transcript box: theAPprofessor.org/podcast-episode-123.html
★ Need help accessing resources locked behind a paywall? Check out this advice from Episode 32 to get what you need! my-ap.us/paywall
Take The A&P Professor experience to the next level!
★ theAPprofessor.org/community
Earn cash by referring other A&P faculty to this podcast:
Tools & Resources
★ TAPP Science & Education Updates: theAPprofessor.org/updates
★ Amazon: amzn.to/2r6Qa3J
★ Text Expander: theapprofessor.org/textexpander
★ Rev.com: try.rev.com/Cw2nZ
★ Snagit & Camtasia: techsmith.pxf.io/9MkPW
★ Krisp Free Noise-Cancelling App: theAPprofessor.org/krisp
★ JotForm (build forms for free): theAPprofessor.org/jotform
★ QuillBot (writing tools): theAPprofessor.org/quillbot
★ The A&P Professor Logo Items: https://www.teepublic.com/stores/the-a-p-professor
Sponsors
★ Transcript and captions for this episode are supported by the American Association for Anatomy | anatomy.org
★ The Human Anatomy & Physiology Society provides marketing support for this podcast | theAPprofessor.org/haps
★ Distribution of this episode is supported by the Northeast College of Health Sciences online graduate program in Human Anatomy & Physiology Instruction (HAPI) | northeast.edu/hapi
Clicking on sponsor links helps let them know you appreciate their support of this podcast!
Follow The A&P Professor on Twitter, Facebook, Blogger, Revue, Tumblr, or Instagram @theAPprofessor
The A&P Professor® and Lion Den® are registered trademarks of Lion Den Inc. (Kevin Patton)
As an Amazon Associate I earn from qualifying purchases. I may be compensated for links to sponsors and certain other links.
Click here to listen to this episode—or access the detailed notes and transcript.